 
| マイケル・W・ハンカピラー |
 |
|
 |
 |
 |
|

[figure 27]

[figure 28]
[figure 29]

[figure 30]
[figure 31]
[figure 32]
[figure 33]
[figure 34]
[figure 35]
[figure 36]
[figure 37]

[figure 38] |
Finally, while
the tools were developed as part of a basic research program to
conduct genome-wide studies, or studies of individual genes, they
have had utility in a variety of other kinds of DNA analyses. I
have listed a few of these here. [figure 27] DNA sequencers, used
not as sequencers but as "fragment analyzers," (to analyze DNA fragments
amplified using PCR techniques) are now the main tool for doing
DNA forensic analysis, either for criminal or paternity purposes
or identification of victims in disasters. At the same time, they
are used to trace the outbreak of bacterial or viral infections.
Therefore, they have had a lot of uses and real-world applications
outside of basic research, and they are beginning to be used in
the diagnosis of clinical problems in the management of people's
health care.
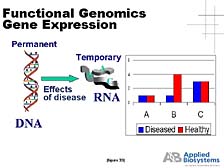
As the importance of sequencing is becoming more recognized, it's important to realize that this is just a start. Sequences give you the basic structure of the genes. What you infer from the sequences is important, but it does not tell us everything about how genes work. [figure 28] What biologists insist on doing is understanding what these genes do, what happens when they work well, and what happens when either the environment or gene structures change and cause gene function to deviate from it should be. And eventually, if there are problems with the genes and the proteins that come from them, what you can do about it from a medical sense.
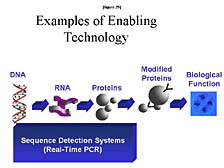
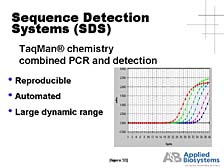
The next couple of tools that I will talk about, which follow from the ability to generate high-throughput sequence information, will touch on some of these functional problems. The first of these is what we call "Sequence Detection Systems." [figure 29] I will explain a little bit about what these are, but predominantly they are tools that are used to look at a large number of individuals, at diversity within DNA, or within a sort of active component of nucleic acids, RNA, to see which of these genes are actually active in one set of conditions or another. The key tool here, which was developed by Kary Mullis and his colleagues at Cetus Corporation in the early 1980's, is a technology called Polymerized Chain Reaction, or PCR. This is a way of making unlimited quantities of a particular gene fragment, or sometimes a whole gene, for isolated chemical or biological analysis. It probably is the defining tool, from a technique perspective, that enabled the revolution in biology over the last fifteen years. Furthermore, PCR really has become an ubiquitous tool for any biology lab. However, it has also quickly branched out from the research lab into a whole host of applications, from forensic analysis (by preparing the samples for analysis) to clinical diagnostics.
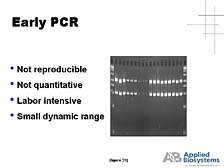
I put this slide up for a variety of purposes, but primarily to point out that one of the applications of PCR was in the identification of individuals, as part of criminal investigations or for disaster purposes. [figure 30] The New York Trade Center bombing referred to here is not the recent one, although it's playing the key role of identifying the victims there. In the first New York Trade Center bombing several years ago, PCR was used in tracking down the perpetrators by analyzing the DNA in the saliva contained on one of the notes taking credit for the bombing. As powerful a tool as it was, PCR in its early days was not a robust analytical process. It was really a way of preparing DNA for subsequent analysis. [figure 31] PCR wasn't a very reproducible process, particularly from a quantitative perspective, and it was a very labor-intensive process. [figure 32] It was not until the mid-1990's, when we developed an automated way of analyzing the DNA concurrently with the production of the DNA by PCR, (what we call a "real-time" system) that it became a robust analytical process that could be applied to quantitative functional problems in the study of DNA and RNA.
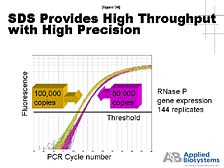
The first utility is what's called "RNA expression," when one wants to compare the amount of RNA being made in a living cell by a particular type of gene under one set of conditions versus another. You need a tool that goes in, pools that specific bit of RNA out of the mix, amplifies it, and then gives you quantitative data. [figure 33] This data tells you how much is there and compares, as this slide illustrates, the amounts of various genes whose relative abundance changes between a disease state and a healthy state. This is one way of getting a clue for which genes are more important in affecting that healthy versus disease state. [figure 34] The ability of our Sequence Detection System to do this with both very high throughput and a high level of precision makes it the gold standard for how to do this in the world today. It's paired with a lot of "DNA microarray technologies" that allow you to look broadly at all the genes and gets some hints as to which ones you really want to focus on for a large number of quantitative studies.
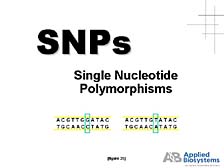
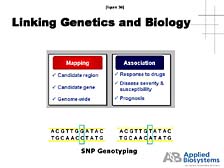
The other application is "genotyping applications" or "Single Nucleotide Polymorphism (SNP) applications." [figure 35] What this boils down to is looking at specific regions of genes and very localized changes from one person's gene sequence in that area to another, to get an indication of where these changes might be important functionally in giving rise to some disease state or some other trait that one is looking at. [figure 36] Here, we really begin to see a link between genetics and biology, because mapping these subtle differences allows us to understand which genes are important in specific functions and how they are associated with various kinds of important health parameters (e.g., response to drugs, disease severity and susceptibility, or the prognosis for treatment based on genetic background). [figure 37] One needs tools, instruments, and automated systems here, too, and interestingly the systems we make use the same fluorescence detection methodology that our sequencers do. But you have to realize the fact that there is a fair amount of diversity within the human genome. While we are all essentially identical at the DNA sequence level, we do differ by approximately 1 out of 1,000 positions in our DNA codes. When you multiply these differences times 3 billion base pairs, we find that individuals differ by a few million base pairs. When it comes to designing assays for all of those differences, it is not a trivial problem to come up with validated analytical tests that cover all of the potential variations. [figure 38] And when you get down to choosing to develop specific assays, the ability to have a hypothesis of which areas of the genome are going to be important and how to design experiments is really going to be the key to utilizing the information that comes out of these sequencing programs. |
|
|